Objects from Rutherford's Life
Crafts
There is no question here of launching into a science course, to explain in detail the ins and outs of Rutherford's experiments.
It is rather a matter of showing, by explaining the conditions under which some of his "DIY" were carried out, the ingenuity of the New Zealand researcher and his desire to always keep things simple.
But through these few examples, it is also beautiful human qualities that stand out; these qualities which make Ernest Rutherford, even today, a figure of laboratory "boss" who deserves to be imitated.
Pictures:
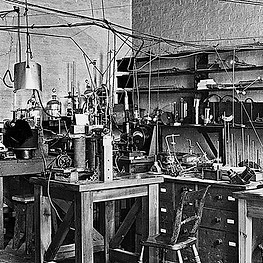
Apparatus used for the fractionation of atoms, in Ernest Rutherford's laboratory in Cambridge Source: Wikipedia
Ernest Rutherford and JA Ratcliffe at the Cavendish Laboratory. Source: Wiley Online Library
If I used the term "crafts", it should not be seen as the slightest desire on my part to denigrate the work of Rutherford and his acolytes. Quite the contrary: the devices imagined and assembled by his teams are truly admirable, all the more so when we look back at the time. Not only were the technical means still rudimentary*, but the field of research in which Ernest was advancing was totally untouched. What the middle school and high school students learn today in a few hours have been identified very gradually by Rutherford and others ... in several years.
*These scientific methods, which combined inventiveness and the search for savings, were described by an English expression: "sealing wax and string science"... which could be translated as a science "made of bits of string". This was in contrast to the experimental techniques of the following decades (from the 1920s and 1930s), which were more commonly referred to as "big science", with particle accelerators in particular.
Moreover, the term "crafts" really corresponds to the principles of Ernest: used to working in deplorable conditions when he began his first research in Christchurch, he then continued in Cambridge to apply the experimental method advocated by JJ Thomson and the majority. heads of laboratories of the time: the “ string and sealing wax physics ”; literally, "string and sealing wax" physics, which required dealing with the simplest components to assemble your research devices. Ernest, who had not forgotten that he was a peasant's son and had lived with twelve brothers and sisters, got along very well with these principles of economy ... and even imposed them on his collaborators when he became him same lab boss.
Finally, I said to myself that if I titled this section "DIY" rather than "scientific experiments on radioactivity", I had perhaps more chances that visitors to this site would not run away.

The wave detector

Photograph of the 1896 Rutherford wave detector shown on page 96 of The collected papers of Lord Rutherford of Nelson, volume I.
Source: Archive.org
Alas, at the same time, an Italian engineer by the name of Guglielmo Marconi was doing the same research and managed to receive a signal from a mile and a half away.
At the request of JJ Thomson , Ernest made a presentation of his apparatus at the colloquium of the British Association which was held in September 1896 in Liverpool . Then he put it aside.
Because during the summer, when he had been in England for less than a year, he had already moved on: JJ's hobby was to study the influence that various radiations could have on the conduction of electricity in gas. At his request, Rutherford looked into the effect produced by ultra-violets, then by X-rays, discovered in December 1895 by the German Röntgen, and finally the "uranic radiation" highlighted by the French Becquerel a few months later.
But he quickly abandoned the subjects proposed by his boss: rather than the target, he was interested in the weapon: JJ continued with the help of other student-researchers his observations on the conductivity of gases, while Rutherford began in mind to understand what these "uranium rays" or "Becquerel rays" could well be.
His first contribution in this field took place in 1898: he demonstrated that there was not just one kind of radiation, but three: alpha, beta and gamma rays. He chose these three names and they are still used today. This is also the case with another word coined the same year by Marie Curie to designate this phenomenon: radioactivity.

The first invention of Ernest Rutherford, this wave detector aimed to capture radio waves at a distance that the young physicist tried to gradually increase.
It was in Cambridge , in 1895 and 1896, that he worked on this device and it was therefore in the Cavendish laboratory, then in the city that he made his tests.
He managed to cover a distance of three-quarters of a mile, between his workplace and the park known as Jesus Green .
The idea was to be able to install a transmitter-sensor system between the coast and the boats, so as to be able to transmit messages on days when the fog made the headlights inoperative.
The search for funding began, Ernest already saw himself living on his royalties, being able to devote himself solely to research and, above all, feeling authorized to marry his fiancée whom he had left in New Zealand.
Plan of central Cambridge: Cavendish laboratory where Ernest Rutherford worked is roughly in the center, between Corpus Christi College and Zoology Museum; Jesus Green is far north. Source: Wikipedia
Plan of central Cambridge: Cavendish laboratory where Ernest Rutherford worked is roughly in the center, between Corpus Christi College and Zoology Museum; Jesus Green is far north. Source: Wikipedia

Electroscopes
Gold leaf electroscope diagram shown on page 16 of the book
Elementary lessons in electricity & magnetism , by Silvanus Phillips Thompson, 1851-1916
Source: Archive.org
An indispensable tool for early radioactivity research, electroscopes required rigorous adjustments to ensure precise measurements.
These demands are at the origin of an anecdote featuring Arthur Stewart Eve in Montreal ; and which gave birth to the first safety rules in the handling of radioactive materials.
To be continued....

First, here is an excerpt from my novel in which I present the beginnings of Ernest Rutherford at McGill University in Montreal:
Throughout his new experiments, he could rejoice in working in such a well-stocked establishment. The main instruments he used were, however, fairly simple and by no means original: exactly identical electroscopes were fitted to the Cavendish in Cambridge or the Curie laboratory in Paris - although Pierre Curie had developed a more precise personal version of them. But unlike what he used to use in England, all the devices Ernest had in Montreal were new and saved him precious time by sparing him hours of repairs and adjustments.
He started - or started again - his measurements with a classic gold leaf electroscope. Designed as early as the 18th century, this instrument had undergone many improvements, but always remained based on the same principle: repulsion between electric charges of the same sign and attraction between charges of the opposite sign. At the start of the operation, the two gold leaves of the device were charged with electricity and therefore repelled each other; if any event led to the formation of electric charges capable of canceling those carried by the leaves, they move closer to each other; by comparing the angle formed by the gold leaves before and after this move, one could assess the importance of the disturbance at the origin of this change in charge. In this case, the phenomena studied by Ernest with this system had been X-rays, ultraviolet rays and finally, uranium rays.
The other device was based on a much improved version of the electroscope: the quadrant electrometer, an instrument developed by Lord Kelvin - a name which, decidedly, seemed to follow Ernest - and which featured a small helix instead of the gold leaves This allowed greater precision and that is why Rutherford had chosen it and associated it with an ionization chamber, so as to constitute a device more suited to the study of alpha rays.
And now I let you discover Arthur Stewart Eve's contribution to working methods in laboratories handling radioactive materials.
As this is a 4-page chapter from my novel, I did not copy the text here: you just have to click on the thumbnail of the first page opposite to open the document in PDF format.
It is only available in french, but you can read the same story, told by Arthur S. Eve himself, as it was published in Nature on March 16, 1905. Its title was "The Infection of Laboratories by Radium". And the text is available on Archive.org.

Arthur Stewart Eve in 1907
(And if, at the end of your reading, you are wondering what the hymn Onward Soldiers of Christ is doing here, go and lend an eye (and an ear) at this page of the site .)
To learn more about Rutherford's electroscope and all the other materials he used in Montreal, you can visit (virtually) the Rutherford collection at the McGill Museum .
There you will find high-definition images of all the display cases in which his equipment is displayed (with detailed explanations and diagrams associated with each object).
For example, showcase E contains the gold leaf electroscope cited here (click on the image opposite, then on the "Cabinet E" tab to see the entire showcase and the corresponding explanatory text).
Isn't it moving to see all these "tinkering", designed and assembled over a hundred years ago and which have led to the in-depth knowledge we now have about the constitution of matter?
(Well, I admit that it only moves the fanatics... of which I am!)
The particle counter
Developed in Manchester in collaboration with Hans Geiger, the purpose of this device was to be able to count alpha particles, one of the components of the Becquerel rays described above.
But why count them? To find out what their nature was.
In the article describing the counting method developed by Rutherford and Geiger, the following paragraph on the first page clearly indicates the objectives of this work:
"The need of a method of counting the a-particles directly without any assumption of the charge carried by each has long been felt, in order to determine the magnitude of the various radio-active quantities with a minimum amount of assumption. If the number of a-particles expelled from a definite quantity of radio-active matter could be determined by a direct method, the charge carried by each particle could be at once known by measuring the total positive charge carried by the a-particles. In this way, it should be possible to throw some light on the question whether the a-particle carries a charge e or 2e, and thus settle the most pressing problem in radio-activity, viz., whether the a-particle is an atom of helium."


An electrical method of counting the number of α-particles from radio-active substances
Ernest Rutherford and Hans Geiger, Proceedings of the Royal Society of London. Series A, Volume 81, Issue 546, Aug 1908.
Received July 17, 1908, Published 27 August 1908
According to Ernest Rutherford's calculations, radioactive materials emit several tens of billions of alpha particles per second. How can we isolate just one of these particles? And once it has been isolated, how can we identify this infinitesimal portion of matter?
On the third page of the same paper from the summer of 1908, the two experimenters explain how they proceeded.
"In our experiments to detect a single a-particle, it was arranged that the a-particles could be fired through a gas at low pressure exposed to an electric field somewhat below the sparking value. In this way, the small ionisation produced by one a-particle in passing along the gas could be magnified several thousand times. The sudden current through the gas due to the entrance of an a-particle in the testing vessel was thus increased sufficiently to give an easily measurable movement of the needle of an ordinary electrometer."


Images :
-
Apparatus used by Rutherford and Geiger. Diagram in their article of August 1908
-
Geiger (left) and Rutherford in front of their apparatus
The active matter, in the form of a thin film of not more than 1 square cm in area, was fixed in one end of a hollow soft iron cylinder which could be moved along the glass tube from the outside by means of an electro-magnet The glass tube was then exhausted by means of a Fleuss pump and, if required, to a still lower pressure by means of a tube of cocoanut charcoal immersed in liquid air.

When the stop-cock was closed, no a-particles could enter the vessel, and the steadiness of the electrometer needle could thus be tested at intervals during an experiment. On opening the stop-cock, a small fraction of the total number of a-particles expelled per second passed through the aperture into the detecting vessel. In practice, it was found convenient to re-arrange the intensity of the active matter and its distance from the opening bot so that from three to five a-particles entered the detecting vessel per minute.
It became difficult to count a number greater than this with certainty, since the needle had not time to come to rest between successive throws.
Once again, the principle is quite simple, explained in this article in Rutherford's usual way, i.e. clearly, succinctly, without excessive technical jargon (and even if you don't know what a Fleuss pump is, you can understand the sequence of operations).
In fact, the point to focus on is the way in which the initial challenge was met: thanks to this fairly basic apparatus, Rutherford and Geiger were able to count alpha particles, reducing the number of particles from ‘several tens of billions per second “ to ”between 3 and 5 per minute’.
As a reminder, an alpha particle corresponds to the nucleus of a helium atom (as Rutherford demonstrated with Thomas Royds). This means that its dimensions are about one millionth of a billionth of a metre. And in 1908, using an assembly of glass elements, two men succeeded in counting these objects belonging to the world of the infinitely small.
The Rutherford-Royds-Baumbach experiment
The Nature of the α Particle from Radioactive Substances.
E. Rutherford and T. Royds [1] , Phil. Mag. 17, 281-6 (1909)
The experimental arrangement is clearly seen in the figure. The equilibrium quantity of emanation from about 140 milligrams of radium was purified and compressed by means of a mercury-column into a fine glass tube A about 1.5 cms. long. This fine tube, which was sealed on a larger capillary tube B, was sufficiently thin to allow the α particles from the emanation and its products to escape, but sufficiently strong to withstand atmospheric pressure. After some trials, Mr. Baumbach succeeded in blowing such fine tubes very uniform in thickness. The thickness of the wall of the tube employed in most of the experiments was less than 1/100 mm ., And was equivalent in stopping power of the α particle to about 2 cms. of air.


As the excerpt from the article presented here, published by Rutherford and Royds in 1909, indicates, the apparatus used to determine the nature of the alpha particles required the construction of a glass tube "sufficiently thin to allow the α particles to escape [...], but sufficiently strong to withstand atmospheric pressure."
Indeed, the particles in question have a very low penetrating power: a few centimeters of air can stop them. How to ensure that they can pass through the necessary glass wall to prevent them from being polluted by gaseous substances present during the reaction? This was made possible by an exploit of a glass craftsman whom Rutherford had called upon: Otto Baumbach, a German living in Manchester, near Victoria University. Thus, Rutherford adds in his article co-authored by Thomas Royds, a young researcher in his team:
"After some trials, Mr. Baumbach succeeded in blowing such thin tubes very uniform in thickness. The thickness of the wall of the tube employed in most experiments was less than 1/100 mm...,"
A glass wall a hundredth of a millimeter thick is a real miracle, which only Baumbach's talent made possible. A talent as exceptional as seeing a physicist, laboratory director, and Nobel Prize winner cite the name of a craftsman in a scientific article. But it was one of Rutherford's principles: to give to each and everyone the honors they desserve.

Pictures:
-
Apparatus crafted by Otto Baumbach and used by Rutherford and Royds to isolate alpha particles. Source: Lemoyne (diagram) and Technician Journey (photograph)
-
Otto Baumbach in his workshop in 1928. Source: " Otto Baumbach - Rutherford's Glassblower", by Alan Gall
The Rutherford-Geiger-Marsden experiment
In 1909, Rutherford one day walked into one of the halls of the physics department he headed at Victoria University of Manchester . He immediately notices that a prism has been moved in the assembly he wanted to use. He then goes into one of those Homeric rages of which he is capable and looks around him for who could have committed such a sacrilege. However, the room where he is is occupied by only one other person: a 19-year-old student named Ernest Marsden. It is obviously him which wipes the exasperated remonstrances of the “Prof” (nickname which Geiger gave to Rutherford). But the young man, by dint of hearing repeat "Did you do that?", ends up answering "No".
Marsden will admit years later that the brevity of his answer was not motivated by any fear on his part in the face of the accusatory eructations with which the Prof stupefied him, but rather by the fact that this ruddy fanatic who addressed him so violently made him want to laugh.
Stopped dead by this "no", Rutherford left the room like a hurricane.
Two hours later he returned. And he apologized to Ernest Marsden for his fury, telling him that he had found the culprit.
The tall scoundrel then pulled out a stool and sat down next to the student. He began to question him on the progress of his research, on his plans for the future... and the two Ernests, separated by twenty years, as well as their position at the two ends of the organization chart of the department. of physics, discussed for hours that afternoon, like two friends


Rutherford then referred Marsden to Hans Geiger , to assist him in developing his particle counter. But the fate of these three men changed abruptly when the youngest pointed out that their experiments were disturbed by the "bounce" of alpha particles on the roughness present on the surface of the glass tubes they were using. Geiger and Marsden therefore had to make some adjustments so as not to be bothered by these alphas moving away from the axis of the beam they wanted to study.
But, as often, Rutherford interpreted this "concern" as an opening to a new line of investigation.
He therefore asked Marsden to check whether these "bounces" of the alpha particles on obstacles moved them away from the axis of the beam only at small angles. The Prof's intuition turned out to be correct: some particles were going straight back.
Geiger and Marsden used for their experiments various metal foil, all of a very great thinness. They then noticed a change in the angles, depending on the metal: the gold leaf, composed of the heaviest metal atoms at their disposal, gave the greatest angles.
That said, such an observation imposed great patience on them: this "backtracking" only occurred for one alpha particle out of 8000.
After enrolling in a course in statistics which seemed essential to him to analyze his data (which greatly surprised the students who saw Professor Rutherford sitting next to them in the lecture room), Rutherford deduced that the atom must have a very dense mass in its center, capable of repelling positive particles that approached it, but that all the rest of the atom was made up of vacuum. With his calculations, he succeeded in demonstrating that the diameter of the atom was 100,000 times greater than that of its central mass. The latter was called "nucleus", on an idea of Niels Bohr, and the distant zone which marked the limits of the atom, was identified as being the region where the electrons of the atom circulated.
Images of the apparatus used by Geiger and Marsden ("gold foil experiment"):
-
Diagram of the original article. Source: Wikipedia
-
3D reconstruction. Source: Wikipedia
-
Simplified diagram of the experiment supplemented by a diagram of the trajectories of the alpha particles through the gold foil. Source: Phylogamous

Ernest Rutherford thus presented in 1911 his model of the planetary atom, which confirmed an idea put forward nearly ten years earlier by the Japanese physicist Hantaro Nagaoka and contradicted the "plum pudding" atomic model established at the same time by JJ Thomson
In 1913, the same Niels Bohr made another remarkable contribution to the Rutherford model, by determining how the electrons behaved around the nucleus. He won the Nobel Prize in Physics in 1922 for this. One frequently speaks of the planetary model of "Rutherford and Bohr".
But that's not all: as for the experiment of Rutherford and Royds mentioned above, this experiment of the bombardment of a sheet of gold by alpha particles benefited from a technological prowess. Indeed, the alphas were detected by the flickering they caused on a screen lined with zinc sulphide. It was therefore necessary to have a circular screen in order to be able to observe all the impacts, whatever the angles of deflection. And it was necessary to be able to rotate the "particle cannon" to also study the influence of the angle of arrival of the particles on the gold leaf. And, finally, the whole had to be emptied of its air, since the alpha particles, by meeting the molecules present in the air, could also be deflected, distorting all the measurements.
Taking into account all these constraints gave rise to the assembly represented in different ways here. And the basic idea for arranging the different parts of the apparatus came from the youngest of the brains involved in the affair: that of Ernest Marsden.
La découverte du proton
As early as 1910, when Geiger and Marsden were bombarding a gold foil with alpha particles, the idea emerged of trying the same experiment on light atoms. It was Charles Galton Darwin who came up with the idea, and Rutherford asked this extremely gifted young mathematician to write a theoretical article on the subject.
The practical part had to wait several years. At first, Rutherford was busy finalising his model of the atom, based on the results of Geiger and Marsden. Then the First World War distracted him from his research, as he was forced to get involved in scientific work related to national defence. True to his serious and committed nature, he did not neglect this mission, but after the United States entered the war in 1917 and his trip to Washington to expose, along with French colleagues, the progress of work undertaken on the Old Continent to detect submarines, he decided, at the end of the same year, to return to his favourite subject. The apathy of ministerial organisations and the obstacles placed in the path of civilian scientists by the military, despite the fact that they were working for them, had taken their toll on his patience.
With the help of his faithful assistant, William Kay, he finally began, seven years after his discussion with Darwin, to study the passage of α particles through hydrogen, nitrogen and other gases. Ernest Marsden was briefly involved in this work once the war was over.
Description of these experiments :
"Rutherford placed a source of radium C (bismuth-214) in a sealable brass container, fitted so that the position of the source could be changed and so that different gases could be introduced or a vacuum produced, as desired. The α particles traversed the interior of the container and passed through a slit, covered by a silver plate or other material, and hit a zinc sulfide screen, where a scintillation was observed in a darkened room. When hydrogen gas was introduced into the container and care was taken to absorb the α particles before they hit the screen, scintillations were still observed. Rutherford posited that as the α particles traversed the hydrogen gas, they occasionally collided with hydrogen nuclei. As Rutherford wrote, this produced “swift hydrogen atoms” which were mostly projected forward in the direction of the α particles’ original motion."
Source : Alpha Particles and the Atom, Rutherford at Manchester, 1907–1919, in Rutherford's Nuclear World, Center for History of Physics at AIP (American Institute of Physics)

Diagram and beginning of the description of the assembly used, taken from the paper Collision of alpha particles with light atoms, I. Hydrogen.
Source : The collected papers of Lord Rutherford of Nelson, volume II, Manchester, page 551
These various experiments led to the writing of four articles in 1919.
The last of the four deals with the disintegration of nitrogen and the expulsion of a hydrogen atom. This particle turned out to be the nucleus of hydrogen.


"From the results so far obtained it is difficult to avoid the conclusion that the long-range atoms arising from collision of a particles with nitrogen are not nitrogen atoms but probably atoms of hydrogen, or atoms of mass 2. If this be the case, we must conclude that the nitrogen atom is disintegrated under the intense forces developed in a close collision with a swift alpha particle, and that the hydrogen atom which is liberated formed a constituent part of the nitrogen nucleus. "
Rutherford's 4th 1919 paper on Collision of alpha particles with light atoms.
Source : The collected papers of Lord Rutherford of Nelson, volume II, Manchester, page 589
In 1920, at the annual congress of the British Association in Cardiff, Rutherford named the particle described in his paper the previous year the ‘proton’. It was to be the first particle to make up the atomic nucleus to be identified... and Rutherford's last major discovery: from the time he took up his post as Director of the Cavendish Laboratory in Cambridge, without ceasing to experiment himself, he devoted himself above all to supervising and inspiring a whole new generation of researchers... several of whom would go on to win Nobel Prizes.


